How To Draw Resonance Structures Ochem
What is Resonance Construction
We can draw two or more Lewis structures for some molecules and polyatomic ions, without changing the position of atoms in the structure. In this case, only the electron distribution differs from 1 structure to another. These structures are called resonance structures or contributing structures. Just, the bodily molecule has an intermediate structure of those possible Lewis structures.
The first step of drawing resonance structures starts with cartoon all the possible Lewis structures. If it has just one Lewis construction, it doesn't have a resonance hybrid.
How to Place the Molecules Having Resonance
Resonance exists only when a Lewis structure has multiple bonds and an next cantlet with at least one lone pair. The general form of resonance is illustrated below. Arrows are used to indicate the shifting of electrons from one resonance structure to some other.

How to Draw Resonance Structures
Lewis Structures
Example 1: COthree 2-ion
Step 1
Calculate the total number of valence electrons from each atom.
Carbon atom = 4
Oxygen atoms (3*half-dozen) = 18
For (-2) charge = 2
** Consider the -two charge at the concluding step (i.e. this molecule has ii actress electrons).
Step 2
When at that place is more than than i type of atom, continue the least electronegative or metal cantlet as the central atom.
Carbon is the key atom in CO3 ii- ion
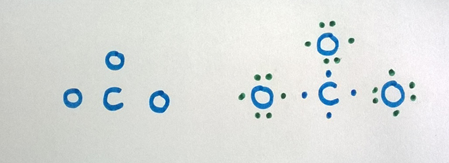
Step 3
Combine each atom with a single bond to the primal atom by contributing ane electron from each atom for the bail.
Footstep 4
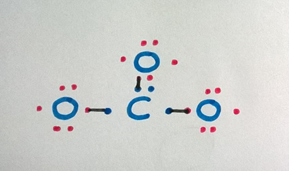
Count the electrons in the valence shell to check if the octet is completed.
The carbon atom needs one more than electron, and each oxygen atom needs ane more electron to consummate the octet.
Stride 5
If not, add some more bonds until all octets are full.
** Get out the not-bonded electrons as dots and draw a single line (-) for a single bail and two lines (=) for a double bond.
If we add 1 bond betwixt the carbon atom and an oxygen cantlet, both carbon atom and the oxygen atom complete the octet.
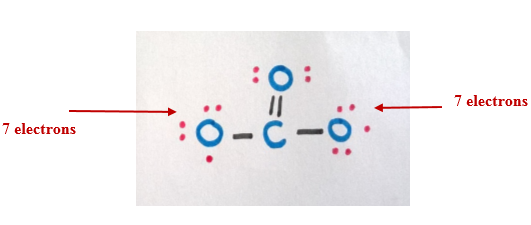
Step 6
Now consider the -two accuse (2 additional electrons). Since the other oxygen atoms have only 7-electrons in their outermost trounce, we can distribute those two electrons among them.
The final structure can be written equally follows.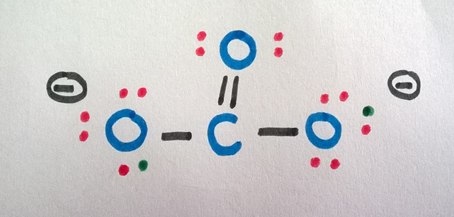
Step 7
Now, we can draw the possible resonance structures as discussed in section one.
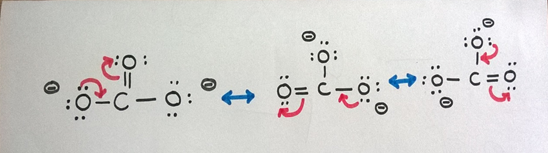
We tin can depict 3 resonance structures for COthree 2- ion as above.
Let's take two more examples to larn how to draw resonance structures.
Example 2: O3 Molecule
We can use the aforementioned process every bit outlined above to obtain the Lewis structure. It gives the post-obit structure, and has a multiple bonds and an next atom with i lone pair of electrons.

Therefore, we can draw resonance structures for O3 molecule as follows.
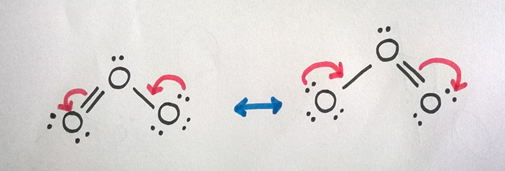
Case iii: Carboxylic Acid
We can obtain the following resonance structures, past following the same steps as mentioned above.
Definitions:
Lewis structure: A unproblematic method of representing the configuration of atoms in a molecule, showing alone pairs of electrons and the bonds between atoms.
References:
Writing Resonance Structures. (n.d.). Retrieved November xv, 2022, from hither
Ch one : Resonance. (n.d.). Retrieved November 15, 2022, from here
A Brief Tutorial on Drawing Lewis Dot Structures. (n.d). Retrieved Nov xv, 2022, from here
Bishop, Thousand. (n.d.). Resonance. Retrieved November xv, 2022, from here

Source: https://pediaa.com/how-to-draw-resonance-structures/
Posted by: aleshirehadly1981.blogspot.com


0 Response to "How To Draw Resonance Structures Ochem"
Post a Comment